Be the first! Get the latest news and updates - Subscribe to our newsletter!
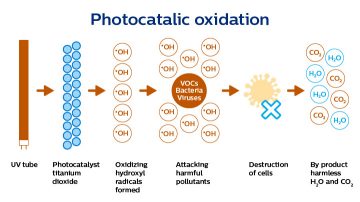
Photocatalytic oxidation is the process of destroying organic substances by oxidation. The technology is well suited to remove low concentrations pollutants (ppb level) from water, air and surfaces.
The technology uses a photocatalytic material that is activated by UV radiation with a wavelength shorter than 380nm to create powerful oxidants (hydroxyl radicals - OH*) at the surface. The substances that need to be removed must come close to the photocatalytic surface. The amount of UV energy to activate the process depends on the photocatalytic material used and the way it is applied on the surface of the carrier.
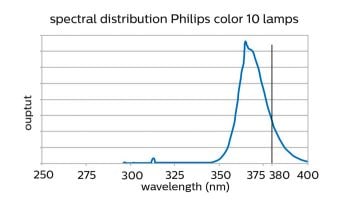
Wavelength
The active material needs to be activated by irradiation with UV energy. The wavelength of this UV energy must be shorter than 380nm. This means UV-A lamps, including Philips color 10 lamps, emit most energy below 380nm should be used. Another option is to use UV-B or UV-C lamps.
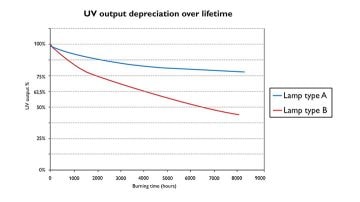
Surface irradiation intensity and homogeneity
It is important that the intensity (mW/cm2) on the surface of the photocatalytic material is sufficiently high to produce enough OH radicals for the complete destruction of the organic target molecules. The required intensity will depend on the construction of the active surface so both shape and photocatalytic material used will have an influence on the energy needed. This means that for each new reactor system, the optimal intensity needs to be verified.
As shown in the graph, different lamp types have different UV degradation curve. UVC lamps for example, will have better UV maintenance vs UVA lamps and will therefore have slower UV degradation.
If part of the surface is either not irradiated or gets lower intensity than it should, the material decomposition will be slower. This will lead to unwanted by-products.
It must be made sure that the end-of-life output of the lamps is still sufficient for complete decomposition of the target molecules.
Additional aspects to consider

Lamp maintenance
It must be made sure that the end-of-life output of the lamps is still sufficient for complete decomposition of the target molecules. The reactor performance will decrease with decreasing irradiance on the catalytic surface.
Lamp temperature
In an air application, the lamp temperature must be checked. The lamp output depends on its wall temperature. This is influenced by the air temperature and the air speed around the lamp. Lower lamp wall temperature may cause a rapid decrease in the lamp output.

Materials used in the reactor
These must be resistant to thew UV radiation. UVC radiation is more powerful and therefore material resistance should be thoroughly checked.
UV-A vs UV-C
UV-A lamps have a faster UV degradation than UV-C lamps. In addition, UV-C lamps will also disinfect by inactivating microorganisms present in the air.
Need support?
We have sales office all around the world. There is always one in your neighbourhood.

